Abstract
Background: Smoldering multiple myeloma (SMM) is an asymptomatic early stage precursor of multiple myeloma. The disease progression to multiple myeloma is an example of series of clonal evolution processes with associated clonal diversity. Therefore, SMM represents a continuous mix of clinically and genetically heterogeneous diseases that cover a broad range of characteristics from monoclonal gammopathy of undetermined significance (MGUS) to multiple myeloma. Identifying the subgroup of SMM with a high risk of progression to multiple myeloma is crucial for any possible early intervention, as well as to spare those with MGUS features from any aggressive interventions. Traditional risk models depended on a static picture of clinical risk factors defined at diagnosis. Recently Mayo group (Ravi et al, Blood Cancer J 2016 Jul;6(7):e454) developed a risk model by factoring in dynamic progression of biomarkers over a year from diagnosis, hence accounting for "rising risk" population at different stages of evolving risk. In this study, we sought to examine the applicability of the newly published risk model for disease progression in our cohort of SMM patients.
Methods: Levine Cancer Institute's plasma cell disorder database was interrogated from January 2012- May 2017 for all smoldering myeloma subjects. Clinical features and outcomes were captured. Evolving change in hemoglobin (eHb) was defined as ⩾0.5 g/dl decrease within 12 months of diagnosis. Evolving change in serum monoclonal protein level (eMP) was defined as ⩾10% increase in monoclonal protein (M) and/or immunoglobulin (Ig) (M/Ig) within the first 6 months of diagnosis (if M ⩾3 g/dl) and/or ⩾25% increase in M/Ig within the first 12 months, with a minimum required increase of 0.5 g/dl in M and/or 500mg/dl in Ig. Time to progression (TTP) was defined as time from SMM diagnosis to diagnosis of symptomatic multiple myeloma or to start of therapy for multiple myeloma, censoring subjects if they died prior to progression. Surviving subjects who had not progressed were censored at last follow up visit. Continuous clinical features were summarized with descriptive statistics, while categorical factors were summarized with frequencies and proportions. TTP was estimated using Kaplan Meier techniques and compared between categorical risk factors using log rank tests. Hazard ratios were estimated using Cox regression.
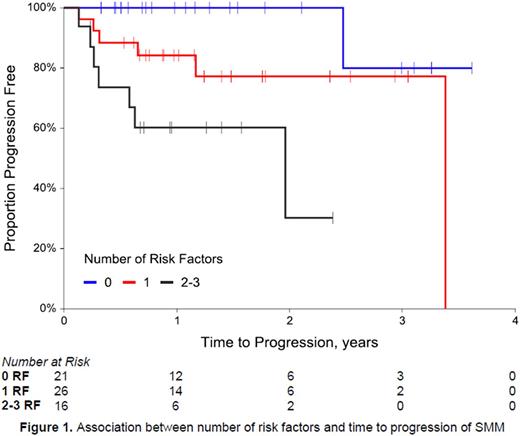
Results: A total of 140 SMM subjects were identified; 66 (47.1%) were male and 84 (60.0%) were Caucasian. Median follow up was 16.7 months (interquartile range 8.3 - 33.6 months). Median age was 67 years (range 31 - 91 years). Ninety-six subjects (68.6%) had IgG subtype. Most subjects had good risk cytogenetics (71.8%), but 17.5% were high risk. Twenty-eight of 90 subjects (31.1%) with readily available bone marrow biopsy results had ≥ 20% marrow plasma cells (PC) at diagnosis of SMM. Thirteen subjects (14.8%) met the criteria for eMP and 52 subjects (54.2%) for eHb. Median TTP was 4.5 years (95% CI: 4.0, N/A), with an estimated 2-year progression rate of 17.7% (95%CI: 10.0%, 25.4%). Evaluating univariate associations, eMP and eHb were significantly associated with TTP (p=0.022 and p=0.005, respectively), while bone marrow PC≥20% was not associated with TTP (p=0.178). When subjects were evaluated as having 0, 1 or 2-3 risk factors (which included eMP, eHb, and bone marrow PC≥20%), 33.3% had 0 risk factors, 41.3% had 1 risk factor, and 25.4% had 2 or 3 risk factors. There was a significant association between number of risk factors and TTP (p=0.003), with the risk of progression in subjects with 2 or 3 risk factors 18.1 times the risk of progression in subjects with zero risk factors. The estimated 2-year progression rate for subjects with 0 risk factors was 0%, compared to 22.8% for subjects with 1 risk factor, and 69.9% for subjects with 2 or 3 risk factors (Figure 1). Data will be updated for dynamic variables including serum free light chain ratios at the time of final presentation.
Conclusions: Our study validates the prognostic value of evolving biomarker-based risk progression model in identifying SMM patients at extremely high risk of progression within two years of diagnosis. Early identification of SMM individuals with a high risk of progression, followed by implementation of evidence-based interventions can slow or prevent progression to advanced stages of the disease, reduce the risk of other complications and improve survival and quality of life.
Voorhees: Oncopeptides: Consultancy; Celgene: Consultancy, Speakers Bureau; Amgen: Speakers Bureau; Bristol-Myers Squibb: Consultancy; Janssen: Consultancy, Speakers Bureau; Novartis: Consultancy; Takeda: Consultancy. Usmani: Novartis: Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Millennium: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Array BioPharma: Honoraria, Research Funding; Onyx: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pharmacyclics: Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; Skyline: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Speakers Bureau. Bhutani: Takeda Oncology: Speakers Bureau; Prothena Therapeutics: Research Funding; BMS: Speakers Bureau; Amgen: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.